I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
This Copy Was Generated On: August 26, 2025
UMMS Biorepository Registration Decision Tree
IRBMED
| Approval Date:
May 22, 2018 9:45 am
This decision tree represents UMMS policy for registration of biorepositories
- funded by a UMMS Department, and/or
- operating in UMMS space, and/or
- directed by faculty with an appointment in the Medical School.
For more information, see Medical School Governing UMMS Biorepositories Policy (level-2 login required) .
Others may use this decision tree to help evaluate whether an REP and oversight committee may be useful for a biorepository or data repository.
Decision Tree - Flow Chart Image
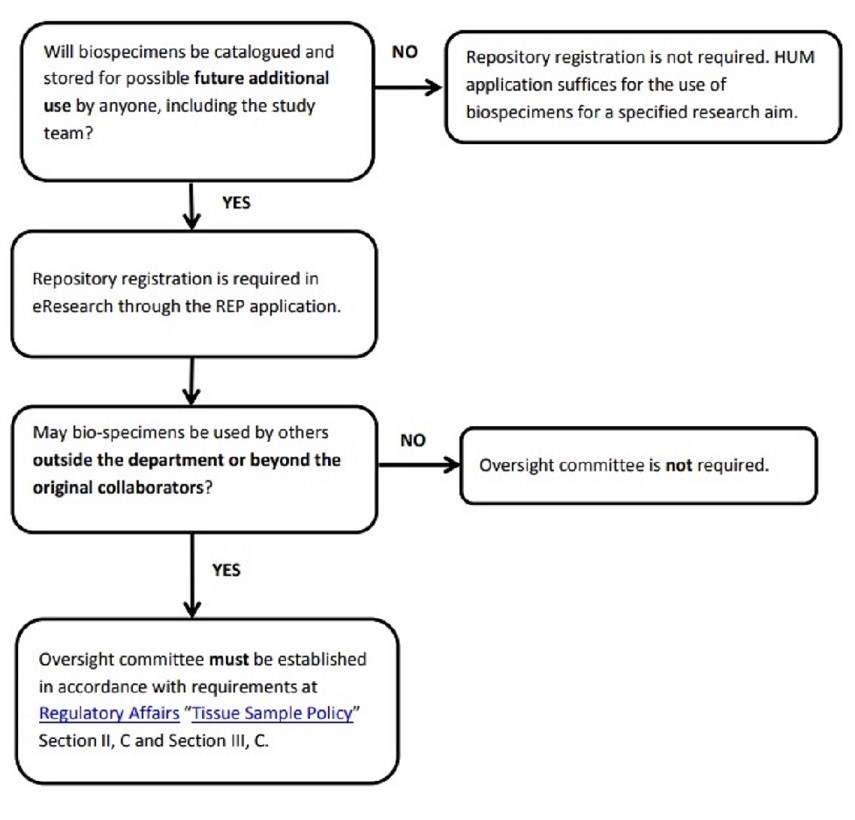
Decision Tree - Flow Chart Text
Question 1
Will biospecimens be cataloged and stored for possible future additional use by anyone, including the study team?
- If no, then repository registration is not required. HUM application suffices for the use of biospecimens for a specified research aim.
- If yes, then repository registration is required in eResearch through the REP application. Now, continue to next question.
Question 2
May biospecimens be used by others outside the department or beyond the original collaborators?
- If no, then oversight committee is not required.
- If yes, then oversight committee must be established in accordance with requirements at UMMS Biorepositories Policy (level-2 login required) Section II, C and Section III, C.
Questions?
Contact us at irbmed@umich.edu or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available in the Personnel Directory, or view the list of Regulatory Teams.
Edited By: larkspur@umich.edu
Last Updated: December 13, 2021 11:00 AM

