I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
Suggested parameters and sets of instructions outlining best practices and standards for accomplishing specific duties.
This Copy Was Generated On: August 24, 2025
eResearch Regulatory Management (eRRM)
IRBMED
| Approval Date:
July 20, 2015 9:15 am
eResearch Regulatory Management (eRRM) is the web-based system that centralizes the review and approval process for Human Subjects Research Applications and IBC Biosafety Registrations.
Reference guides
There are many helpful step-by-step guides on using eResearch under ITS Regulatory Management Reference Materials, including
- PI & Study Team Quick Reference Card
- Logging into eResearch
- Creating an Application for a New Study
- Preparing a Study Application for Submission
- Working with Documents (Uploading Editing, Comparing and Deleting)
- See also IRBMED Document Revision guidance
- Submitting a Completed Application – PI only
Other guidance on using eResearch Regulatory Management includes
- ITS Public KnowledgeBase #eRRM and #eresearch
- ITS Regulatory Management Frequently Asked Questions
- Glossary of eResearch Terms
- Pre-application Checklist–Things to have, information to gather before starting a submission
General workflow
Anyone with an eResearch login can create a new study application. However, ONLY the listed PI can do the initial “Submit” for an application.
The system leads study teams through a “smartform” from one system-required section to another. Always start at a system-required page (page 01 or, if you use the “Jump To:” menu, a page shown on the menu in black type rather than in gray italic type). Fill out each page as it comes to you. i.e. After filling out the study team, project summary, &c. on page 01, use the “Continue” button and let the eResearch ‘system logic’ lead you through all other system-required pages.
TIP: If you find yourself on a non-system-required page, DO NOT use the “Continue” button to leave it: in this case you may NOT be led to the next system-required page.
If you would like to make your IRB staff aware of an application before we receive it – even while it’s in Pre-Submission – you can contact us directly. IRB staff can see any pages filled out for an application at any point after the study team Creates New Study, fills out page 01, and hits “Continue” to go to page 01-1.
Before initial “Submit” becomes available to the PI, study team members must Accept Role, except
- not required for “Administrative Staff” roles
- On Exempt human subjects research applications, required only for “Faculty Advisor” role
- “Not Regulated” applications have no Accept Role requirement
One of the main reasons for this requirement is to facilitate the University tracking potential conflicts of interest.
When the listed PI “Submits” the application, if applicable it routes first to non-IRB UM research units (including Conflict-of-Interest, Research Pharmacy, etc.). IRB receives the application only after other required system approvals are in place.
How to know where an application is in its lifecycle
If an application is with the study team
- Study team members see it in “My Inbox”. There are several headings on your homepage – you may need to scroll down. “Require Action by Study Team” is the “catch-all” heading – anything that appears in the higher tabs ALSO appears here.
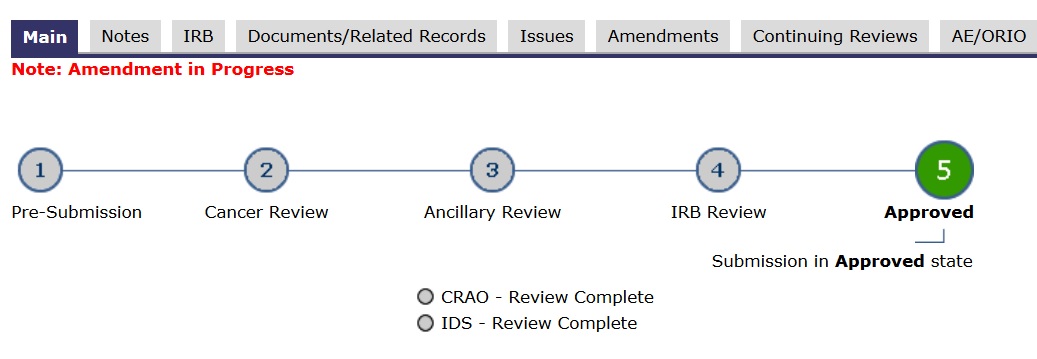
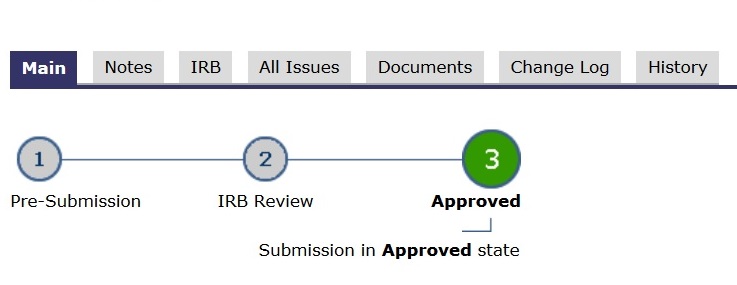
- In the application’s main space, the “subway map” (lines and circles) shows a RED dot. Red means study team action.
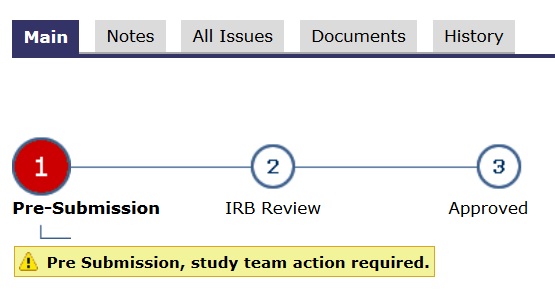
If an application is with a U-M oversight committee (IRB, COI, IDS, etc.)
- Study team members can see it by clicking to their “In Progress” tab (next to the “My Inbox” tab).
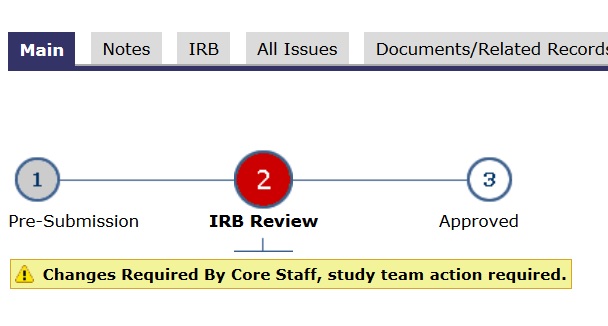
- In the application’s main space, the “subway map” (lines and circles) shows a YELLOW dot. Yellow means committee review.
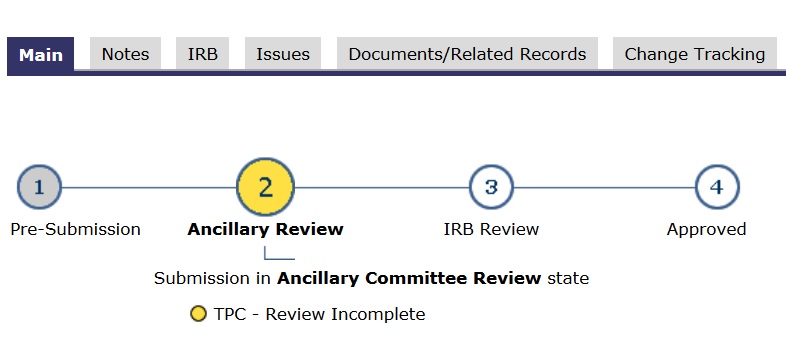
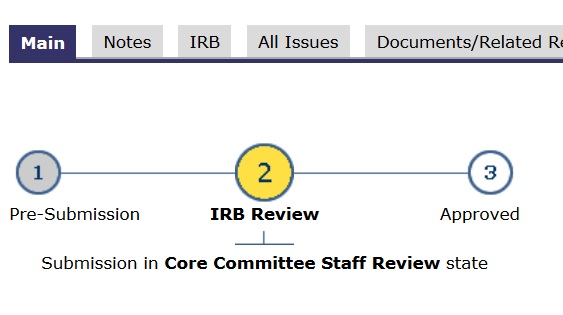
If an application is finalized, “ready-to-go”
- Study team members can see it by clicking to their “Approved” or “Exempt and Not Regulated” tab (farther to the right from the “My Inbox” tab).
- In the application’s main space, the “subway map” (lines and circles) shows a GREEN dot. Green means go.
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available in the Personnel Directory, or view the list of Regulatory Teams.
Edited By: [email protected]
Last Updated: June 9, 2021 12:00 PM

