I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
Suggested parameters and sets of instructions outlining best practices and standards for accomplishing specific duties.
This Copy Was Generated On: September 17, 2025
External (non-UM) Study Team Members
IRBMED
| Approval Date:
May 12, 2025 9:30 am
Overview
Institutional Review Boards have a role in assessing an investigator’s (or, a study team member’s) qualification in conducting the human subject research activities proposed in a protocol or an IRB application. IRBMED review and approval process includes evaluating study team members’ qualifications and appropriate U-M affiliation(s). IRBMED oversight applies to the study team members affiliated with U-M in accordance with IRBMED jurisdiction (outlined in IRBMED Standard Operating Procedures). For study team members that are not affiliated with U-M, appropriate IRB oversight must be established prior to conducting any human subject research activities. IRBMED may choose to extend its oversight to study team members not otherwise affiliated with an institution having an IRB or whose institution is not otherwise a performance site in the research. This guidance outlines the current IRBMED process to identify these external study team members, document them in the IRB application, and work with IRBMED in establishing appropriate IRB oversight.
See also U-M Human Research Protection Program (HRPP) Operations Manual Part 5 (particularly, V. UNAFFILIATED INVESTIGATORS)
Process
External Collaborators: The study team members who are not affiliated with University of Michigan (U-M) but are conducting non-exempt research with human participants on behalf of the U-M. Their institution or organization is not otherwise a performance site.
NOTE: Generally, Active Emeritus Faculty, Adjunct Faculty, Visiting Faculty, Visiting Scholars, Visiting Graduate Students, International Visiting Scholars and Volunteers vetted through Volunteer services count as affiliated with U-M during the term of their sponsorship/appointment. Contact your IRB (IRBMED or HSBS) for confirmation.
I. Identifying external (non-UM) study team members
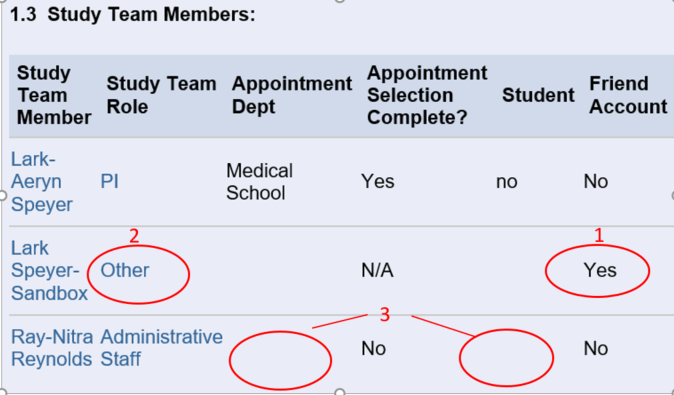
Section 01 of the IRB application (eResearch Regulatory Management) is designed to capture information regarding the study team members. The non-UM study team members are usually identified in various ways:
- The U-M study team self-identifies an individual as an external collaborator.
SPRING 2025 – a new required question in Section 01 prompts listing the names of all non-UM study team members. - A study team member is listed with a Friend Account
- A study team member is pre-identified with “Other” as the study team role
- A study team member is not a student and has no Appointment Department selected
II. IRB Oversight
Generally, the human subject research activities performed by an external study team member are overseen by the institution they are affiliated with.
- If the external study team member’s human subject research activity is already covered by another IRB, there is no need to list them in the UM IRB application. Instead, the site may need to be listed in Section 03-1 of the eResearch application as a participating site. Note that this type of scenario (where the external site is engaged) may trigger Single IRB (sIRB) requirements. Contact IRBMED for guidance on sIRB requirements.
- If the external study team member’s human subject research activity is NOT covered by another IRB and the U-M study team would like IRBMED to consider providing IRB oversight, follow “Managing External Collaborators” guidance from IRB-CSP “References and Resources” heading.
Resources
- U-M Human Research Protection Program
- Operations Manual Part 5
- Authorization Agreements Process
- Single IRB-of-Record (sIRB) Process
- IRB Coordinated Services and Practices (CSP) unit, “Resources and References” heading: Documents on managing external collaborators
- OHRP regulations 45CFR46.109(a) and (d)).
- OHRP guidance “Engagement of Institutions in Human Subjects Research (2008)”
- FDA guidance “IRB Responsibilities for Reviewing the Qualifications of Investigators,…. (2013)”
- ITS eResearch Regulatory Management Training Resources and Reference Materials
- “Adding a Study Team Member”
- “Obtaining a Friends Account”
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available at the IRBMED website.
Edited By: [email protected]
Last Updated: May 12, 2025 9:30 AM

