I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
Suggested parameters and sets of instructions outlining best practices and standards for accomplishing specific duties.
This Copy Was Generated On: September 18, 2025
UMMS Biorepository Registration Decision Tree
IRBMED
| Approval Date:
May 22, 2018 9:45 am
This decision tree represents UMMS policy for registration of biorepositories
- funded by a UMMS Department, and/or
- operating in UMMS space, and/or
- directed by faculty with an appointment in the Medical School.
For more information, see Medical School Governing UMMS Biorepositories Policy (level-2 login required) .
Others may use this decision tree to help evaluate whether an REP and oversight committee may be useful for a biorepository or data repository.
Decision Tree - Flow Chart Image
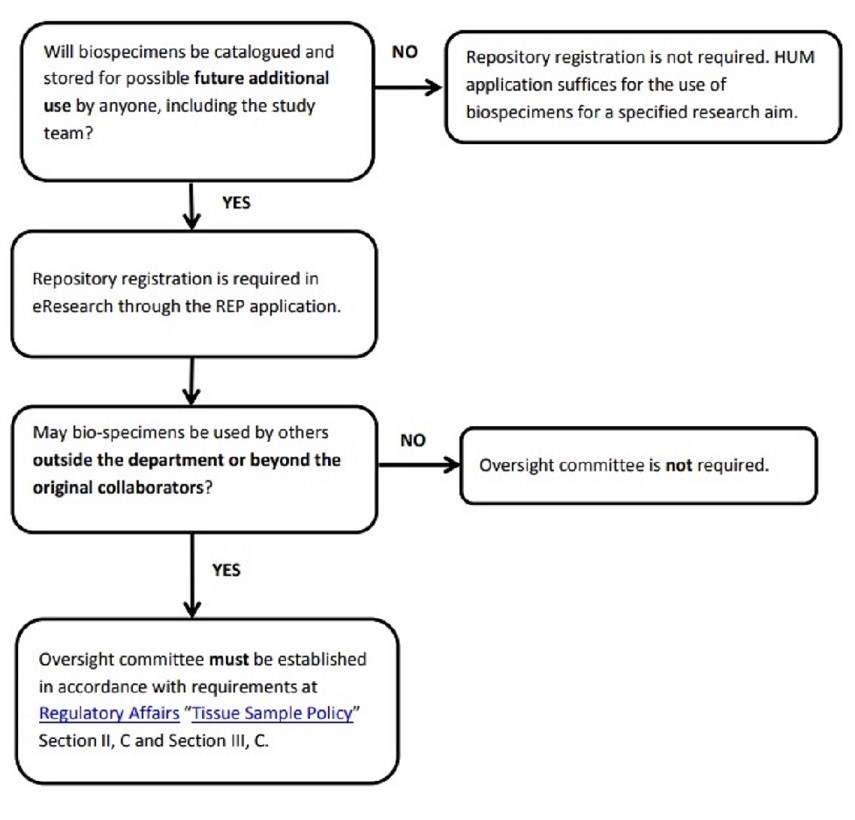
Decision Tree - Flow Chart Text
Question 1
Will biospecimens be cataloged and stored for possible future additional use by anyone, including the study team?
- If no, then repository registration is not required. HUM application suffices for the use of biospecimens for a specified research aim.
- If yes, then repository registration is required in eResearch through the REP application. Now, continue to next question.
Question 2
May biospecimens be used by others outside the department or beyond the original collaborators?
- If no, then oversight committee is not required.
- If yes, then oversight committee must be established in accordance with requirements at UMMS Biorepositories Policy (level-2 login required) Section II, C and Section III, C.
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available in the Personnel Directory, or view the list of Regulatory Teams.
Edited By: [email protected]
Last Updated: December 13, 2021 11:00 AM

