I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
Informational tools to help guide you through specific tasks.
This Copy Was Generated On: September 14, 2025
IRBMED Review Fees
IRBMED
| Approval Date:
June 19, 2023 10:00 am
IRBMED review fees are charged to industry sponsored contracts to underwrite a portion of the administrative costs necessary to conduct IRB review, issue IRB Board approvals, and maintain oversight of a research study. Also, IRBMED charges review fees in association with single IRB (sIRB) reviews for multi-site research when IRBMED is the IRB of Record for the study. Even with this limited recovery, the Medical School continues to heavily subsidize the costs of these reviews.
This fee structure dates to July 1, 2021.
Questions about IRB Review Fees should be directed to:
- IRBMED review — IRBMED
- Contract negotiation — Clinical Trials Support Units or your local research administrator
- General sponsor applicability and reflecting IRBMED Fees/recovery in your sponsored project budget — Grant Services & Analysis Office
Industry-Sponsored Research
IRBMED fees are charged to each new sponsor contract associated with IRBMED review. Reviews of routine amendments, adverse events, and continuing reviews are included in this IRBMED fee and are not subject to additional IRBMED recovery. The IRBMED fee applies to all applicants meeting the criteria listed below, regardless of the originating school or college. Fees will be assessed after the contract is executed, an award is established in eResearch Proposal Management (eRPM), and IRBMED review is completed.
Of note: Medical School studies with contracts negotiated prior to this announcement and awarded after July 1, 2021, will be charged at prior rates. Previously awarded contracts will not have additional fees applied.
IRBMED Review of U-M as a Participating Site
A one-time IRBMED review fee of $3750 encompassing review of the initial submission, amendments, adverse events, and continuing reviews/termination is charged under the following conditions:
- Sponsor is an industry/for-profit entity
- A new contract is executed for a study (including those affiliated with an existing, approved IRB application)
- IRBMED is the IRB of Record for U-M’s participation in the study
- Human research is indicated in the contract
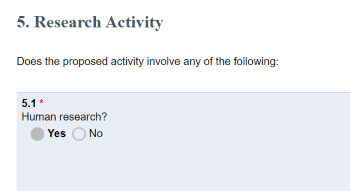
- PAF question 5.1 Human Research = YES
IRBMED Oversight is Ceded to a Commercial IRB
A one-time IRBMED fee of $1300 is charged for review of studies ceded to external IRBs, if the following conditions are met:
- Sponsor is an industry/for-profit entity
- A new contract is executed for a study (including those affiliated with an existing ceded study)
- An external IRB (commercial) conducts the regulatory review
- A commercial IRB is identified as IRB of Record
- PAF question 5.1 Human Research = YES
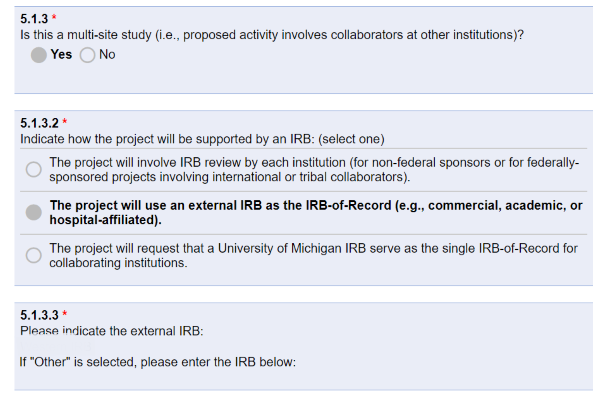
- PAF question 5.1.3.2 = Project will use an external IRB as the IRB-of-Record
Multi-site Research when IRBMED is the Single IRB (sIRB)
Use of a single IRB model (sIRB) may be required by grant or contract for cooperative multi-site research. IRBMED must be informed of this requirement at the time of grant application or contract negotiation in order to assess the circumstances, prepare a budget for inclusion in the grant/contract, and execute Reliance Agreements between institutions. When IRBMED agrees to function as the sIRB for the research, IRBMED will charge the following fee:
- $62.66/hour through service unit billing as work is completed on the project
Budgeting IRB Fees
The fixed, negotiated amounts for the Industry-sponsored agreements where IRBMED reviews ($3750) and for IRBMED ceding to a commercial IRB ($1300) are complete rates which include all UM recovery. This means they are treated as direct costs and are not assessed F&A recovery. Please exclude these amounts when calculating the F&A recovery in your budget.
When IRBMED serves as the sIRB, the $62.66/hour does NOT include F&A recovery and should be listed in the budget as a service of others and be assessed F&A in your budget calculation.
How Fees Will Appear on your Account Statement
The industry-sponsored agreements for IRBMED review and ceding to a commercial IRB will appear as two charges that equal the total fee assessment. They will be charged at the same time and only once for the project.
The sIRB services will appear as a service unit billing (SUB) and will be charged throughout the life of the project as services are assessed.
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available in the Personnel Directory, or view the list of Regulatory Teams.
Edited [email protected]
Last Updated: June 19, 2023 at 10:00 AM

