I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
High-level program plans that outline the general goals and acceptable procedures for a unit.
This Copy Was Generated On: August 12, 2025
Statement of Practice: IRBMED Finalization of Study Documents
| Approval Date:
February 12, 2020 1:30 pm
This document replaces: ‘Approval Dates on Consent Documents and Recruitment Materials’ Statement of Practice.
Statement of Practice
IRBs are expected to review all the research documents and activities that bear directly on the rights and welfare of the subjects of proposed research. This includes consent documents and processes, as well as the methods and materials that investigators propose to use to recruit subjects. These are evaluated before initial IRB approval and as part of continuing review, as applicable.
IRBMED finalizes the informed consent documents and the recruitment documents uploaded in IRB application with an approval date and, if applicable, an expiration date. The study team may add HUM numbers to other subject facing documents. The study team should add HUM# and U-M PI contact information to Emergency cards, unless sponsor formatting requirements prohibit this.
Although other study-related documents (such as the protocol, survey instruments, and other supporting materials) are part of the IRB review, IRBMED does not finalize them. However, per the study team request, these documents can be referenced in the “Supporting Documents” section of the IRB approval letter (in initial applications and amendments fill out 44.2 of the IRB application). When finalizing consent documents and recruitment materials, the finalize “Approval Date” is assigned in accordance with the date that the application was either “Approved” or “Approved with Contingencies.” This determination is made by the Board or by an Expedited Reviewer.
Note: Currently, IRBMED electronic system does not support finalizing certain types of documents (such as, PowerPoint presentations, JPG files, videos, etc.). This mostly occurs with the recruitment documents. For these types of documents, the study teams should indicate the IRB application (HUM) number within the body of the document.
New Study Applications
For new study IRB applications that are approved with contingencies, study activities may not begin until all outstanding contingencies have been met and verified with the IRBMED. Once verified, IRBMED will issue the IRB approval letter. At that time, informed consent documents and recruitment materials will be finalized (when possible).
For new study IRB applications that are approved with no contingencies, consent documents and recruitment materials will be finalized (when possible) after issuing the IRB approval letter.
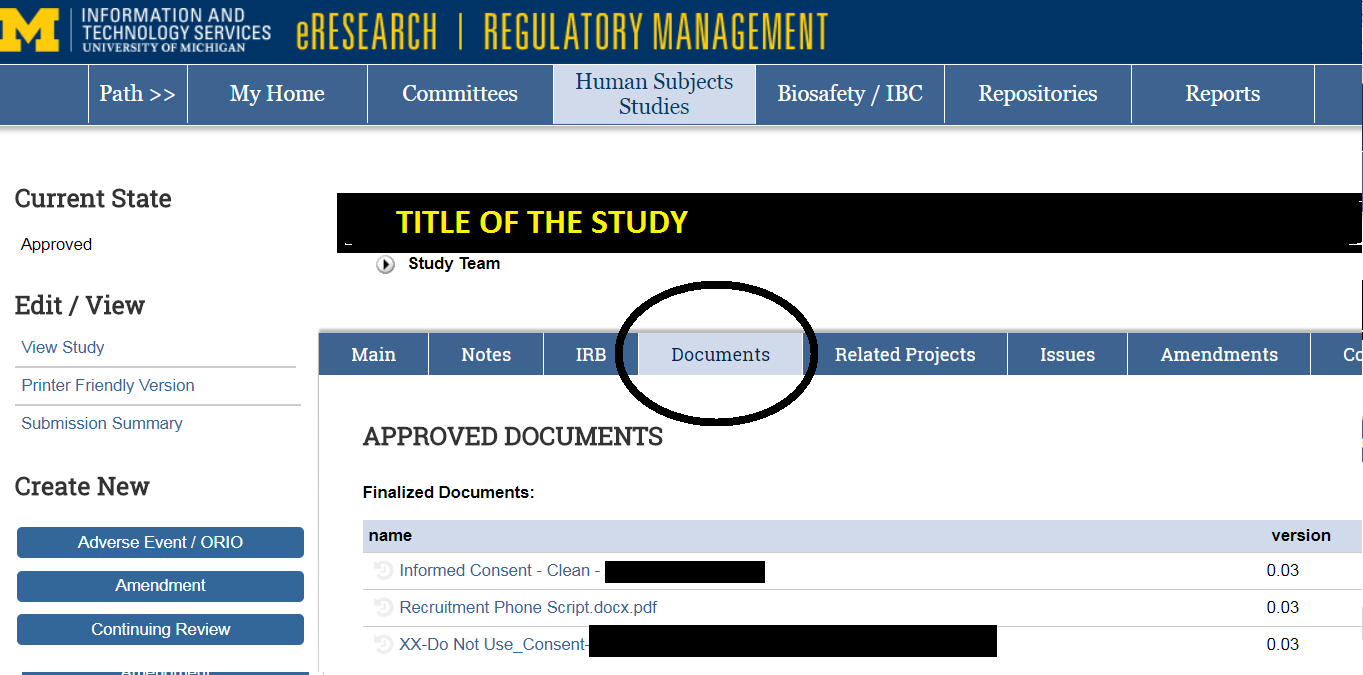
Finalized documents are available under the “Documents” tab of the main work-space of the IRB application. See example below.
Scheduled Continuing Review of Amendment Applications
At the time of scheduled continuing review (SCR) or Amendments, approval dates (and, if applicable, expiration dates) are updated for all relevant documents. Study team members should continue utilizing the most recently approved informed consent documents and recruitment materials until the new/updated documents and materials are issued.
After subject recruitment permanently ends at U-M, recruitment materials are no longer in use and therefore, they will not be finalized. Similarly, informed consent documents will not be finalized after study interaction/intervention ends at U-M.
Exceptions
Contact IRBMED for guidance related to any exceptions: (I) In the event that the study team makes an administrative error regarding the status of the study. (II) If there is a situation where the above process does not address the specific need of the study team.
Examples
General example of IRBMED finalize:

A screenshot of “Documents” functionality where approved documents are located
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available at our website.
Edited By: [email protected]
Last Updated: April 28, 2025 10:15 AM

