I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
Suggested parameters and sets of instructions outlining best practices and standards for accomplishing specific animal care and use research duties.
This Copy Was Generated On: July 11, 2025
Guidelines on the Preparation of Injectable Substances and Agents Administered to Animals
Unit for Laboratory Animal Medicine
| Approval Date:
March 21, 2025 12:00 am
This guideline outlines the expectations for the preparation of injectable substances and agents intended for use in animals.
Glossary Definitions
Pharmaceutical Grade
A drug, biologic, or reagent that is approved by the Food and Drug Administration (FDA) for use in humans or animals or for which a chemical purity standard has been established by the United States Pharmacopeia-National Formulary (USP-NF), or British Pharmacopeia (BP).
These standards are used by manufacturers to help ensure the products are of the appropriate chemical purity and quality, in the appropriate solution or compound, to ensure stability, safety, and efficacy. The FDA maintains a database listing approved commercial formulations for human drugs (the Orange Book ) and veterinary drugs (the Green Book). For chemicals, a certificate of analysis is usually available upon request.
Non-Pharmaceutical Grade
Chemicals or compounds that do not meet or exceed requirements of USP/NF/BP and may have higher levels of impurities that can introduce unwanted variables or toxic effects. Sterile pharmaceutical grade preparations that are reconstituted, diluted, mixed or have other substances added are also considered non-pharmaceutical grade.
Diluent
An agent that dilutes or renders an active compound less potent or irritant. Example – 0.9% sterile saline or sterile water.
Agent
Agents are drugs intended for use explicitly as an anesthetic, analgesic, sedative, tranquilizer, and/or neuromuscular blocking agent (NMBA).
Substance
Substances are all chemicals, drugs, experimental compounds, vehicles, etc. applied in or on an animal as part of the research activities, other than drugs used as anesthetics, analgesics, sedatives, tranquilizers, and/or neuromuscular blocking agents (NMBA).
Preparing Substances and Agents to be Administered to Animals
- Pharmaceutical Grade drugs should be purchased in sterile, rubber topped vials in the smallest volume available that is appropriate for the intended use.
- More information on the use of non-pharmaceutical grade substances or agents in animal can be found in the Policy on the Use of Non-Pharmaceutical Grade Drugs
- Prior to preparation, review the EHS safety findings in eRAM to determine if a specific substance or agent must be prepared in a chemical fume hood or biological safety cabinet.
- Prepare all agents and substances in a manner that ensures the final product is sterile prior to being used in an animal.
- Calculate the weight of the agent or substance needed to prepare the desired volume at the necessary concentration. See Appendix A for example pharmaceutical calculations.
- In a sterile container, dissolve the measured amount of substance or agent in the appropriate volume of diluent.
- Whenever possible, use a sterile, pH balanced (6.8 – 7.2), osmotically balanced (approx. 300 mOsm), pyrogen-free diluentlabeled for injection for humans or animals (e.g. 0.9% Sodium Chloride, Sterile water.
- For non-aqueous formulations, the IACUC may request additional information describing assurances for sterility, potential adverse consequences, etc.
- As needed, adjust pH close to physiologic pH (7.4).
- Dissolve the formulation in an appropriate solvent until particles are not visible. Filter through a 0.2 um filter into a sterile container (a sterile glass vial with a crimped rubber closure is recommended).
- Discard any solutions or formulations that show signs of contamination, breach of sterility, or compromised integrity (e.g., cloudiness, precipitation, order, visible growth, color change).
Dilution of injectable drugs intended for clinical purposes
- Several commonly used drugs (e.g., carprofen, ketamine, xylazine) are readily available in commercially produced formulations for human and veterinary clinical use. These products often require dilution to facilitate accurate dosing in small species like rodents.
- All solutions used for dilution must be pharmaceutical grade and intended for injectable use in humans or animals (e.g. sterile 0.9% Sodium Chloride or sterile water). These fluid diluents must be in sealed, rubber topped vials or bags and must be used within the expiration date. Fluids intended for other laboratory use are not appropriate diluents for these drugs.
- See Appendix B for acceptable and unacceptable diluents.
- Drugs and diluents should be placed into a sterile, rubber stoppered vial and labeled with the following information:
- All names of drugs in diluted mixture. Write drug names in full, do not abbreviate.
- Concentration of drug mixture
- Preparation date and expiration date
- The expiration date for the diluted solution is determined by the earliest expiration date of any of its component drugs or diluents. (Manufacturer’s expiration date for the vial or the vial’s beyond-use-date)
- All names of drugs in diluted mixture. Write drug names in full, do not abbreviate.
- Store solutions according to the manufacturer’s directions per label.
Appendix A: Pharmaceutical Calculations for Diluting and Combining Drugs
Appendix B: Acceptable and Unacceptable Diluents for Clinical Injectable Drugs
Acceptable Diluents
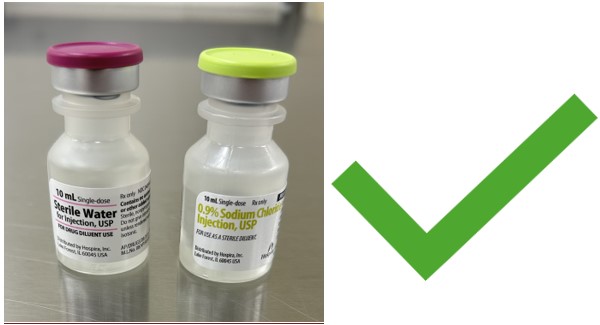
Unacceptable Diluents

References
- Blood DC, Studdert VP. 1999. Saunders comprehensive veterinary dictionary. London ; New York: WB Saunders.
- Lake T. 2003. Dosage calculations for veterinary nurses and technicians. Edinburgh ; New York: Butterworth-Heinemann.
- Matthews, K. and Taylor, D., Assessment of Sterility in Fluid Bags Maintained For Chronic Use; J. American Association for Laboratory Animal Science, Vol. 50, No. 5, Pages 708-712, September 2001.
- Plumb DC. 2005. Plumb’s veterinary drug handbook. Stockholm, Wis. Ames, Iowa: PharmaVet ; Distributed by Blackwell Pub.
- Office for Animal Care and Use, NIH. Updated February 19, 2009. (http://oacu.od.nih.gov/)
Questions?
Questions or concerns about the content of this document should be directed to the Unit for Laboratory Animal Medicine (ULAM) at (734) 764-0277 or [email protected].

