I'm looking for information about
Can't find what you're looking for? You may need to login to see more documents
High-level program plans that outline the general goals and acceptable procedures for a unit.
This Copy Was Generated On: July 19, 2025
Statement of Practice: Document Revision Guidance, Naming Convention, and Version Control
IRBMED
| Approval Date:
October 15, 2021 8:30 am
To assure that study teams are utilizing the most recent IRB approved versions of study documents when making modifications at the time of amendment or when addressing requested changes by IRBMED staff or reviewers, IRBMED has standardized practices for naming convention and version control. The standardized practices, further detailed below, include procedures for:
- Accessing the most recent IRB approved clean version of the consent from the eResearch application in Section 10-1 (Word version)
- Providing a ‘tracked changes’ version of study documents for review
- Applying the standard naming conventions for study documents into eResearch
I. Uploading Consent Documents into eResearch
Upload initial documents for IRB review into each appropriate section of the new study application. This will become the document’s ‘tracked’ stack. Track all modifications to documents after the PI submits the application, even within a new application.
For amendments modifying study materials, upload a tracked version of the document to eResearch. Ensure any subtitle & version # in the document (e.g. footer) are updated.
Maintain one ‘tracked stack’ of documents in eResearch for each (for studies with multiple consent documents). Each updated/tracked version for a given document should be stacked via the ‘Upload Revision’ function into its existing ‘tracked’ stack. Only ‘Add’ new document stacks for entirely new documents.
Note: Documents uploaded into section 44 may not require both tracked & clean stacks. Please work with IRBMED Staff when making revisions to these documents.
Follow the standard naming convention outlined below in Section IV. Updating the stack title is not required, and actual document title (i.e. filename) need not match stack title.
Do not delete any documents or stacks of documents from eResearch; these are retained for historical reference purposes.
Do not alter or remove the consent/assent cover page in the IRB-maintained “Clean” stack(s) in 10-1.1. This page is “silent”, meaning it will disappear during eR document finalization.
Do not use documents stored outside of eResearch as these may be previous versions instead of the latest IRB approved version. IRBMED Regulatory Staff verify the correct document was used via the document’s cover page and the Microsoft Word ‘Compare’ function as an initial step in their review.
II. Document Revision
Microsoft Word Documents
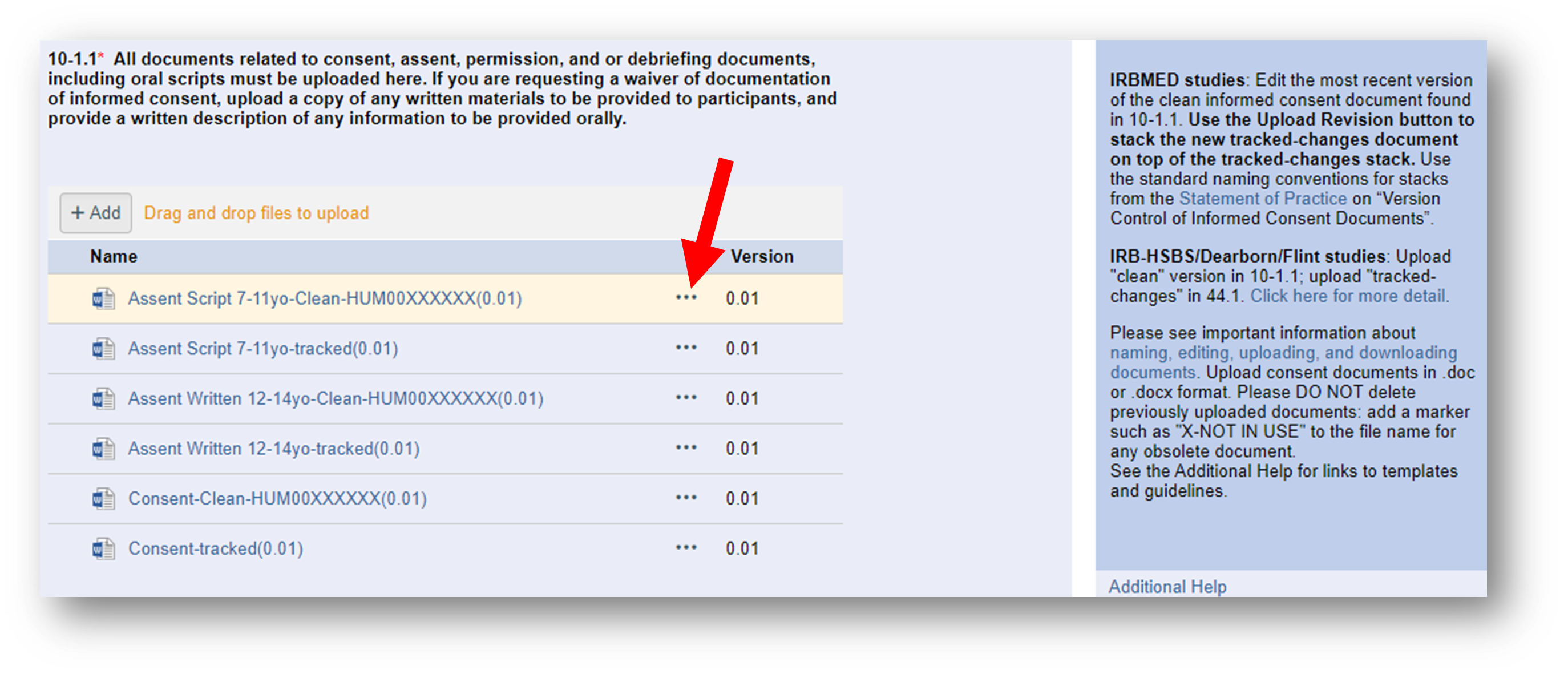
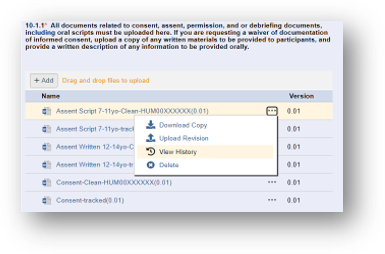
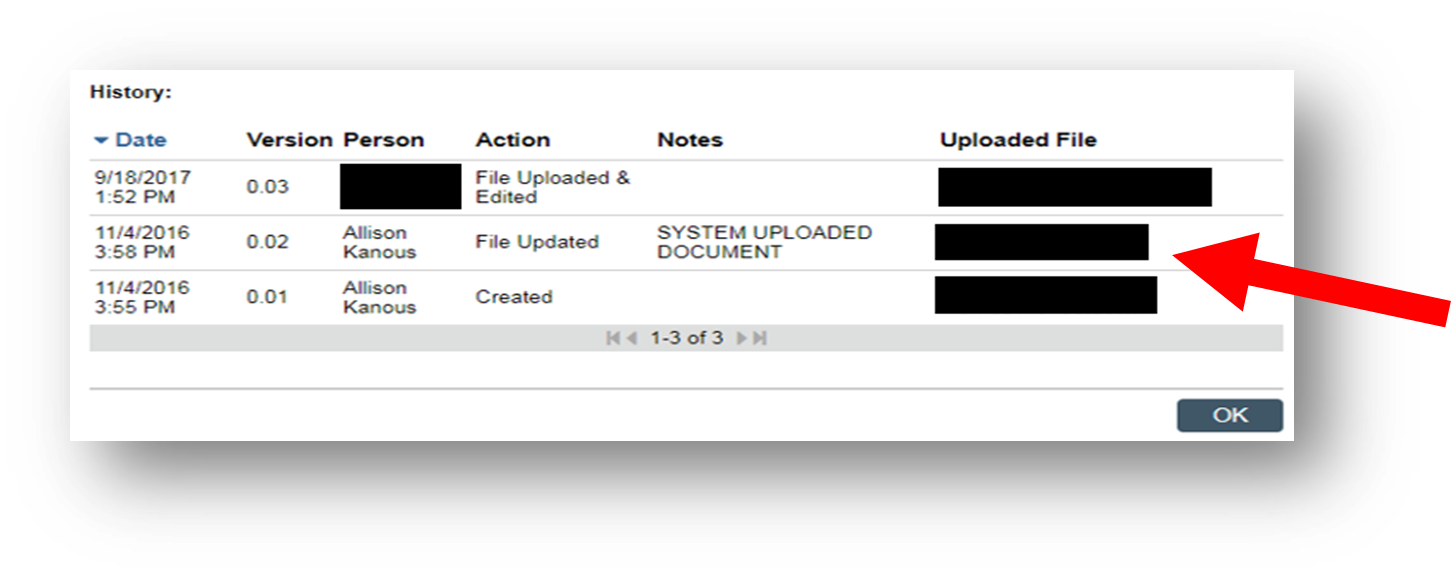
1. Download the last approved “Clean” Word version from eResearch. Locate the most recently approved clean version by clicking on the ellipsis (three dots) then selecting View History.
Sometimes an irrelevant version is inadvertently uploaded into the Clean stack: using the document stack Resource History ensures you download and make revisions from the latest approved Clean Word version
2. As a best practice, “Save” the download with a filename including “-tracked.”
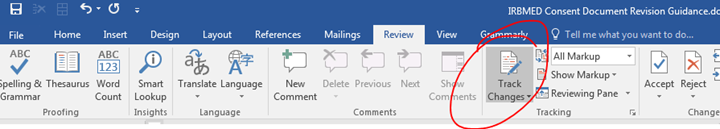
3. Revise with Microsoft Word’s Track Changes active.
4. Upload into the same document’s ‘tracked stack’ in eResearch.
Versions to provide by document function
For informed consent documents, provide only a ‘tracked-changes’ version. IRB staff will ‘Accept All Changes,’ and manage the ‘clean’ stack. See also section IV below.
For protocol documents and test article investigator’s brochure (IB), provide
- a clean version and
- a tracked/redlined version and/or a comprehensive Summary of Changes (SOC) for review.
The SOC may be a separate document stack, or embedded in the new draft.
For recruitment documents (flyers, letters, scripts, etc.), data collection forms (surveys, questionnaires, case report forms, etc.), and other types of documents (including but not limited to screenshots of mobile interfaces, ‘Dear Doctor’ letters, wallet cards, reminder letters, etc.) ‘clean’ and/or ‘tracked-changes’ versions are usually acceptable. Study team is responsible for providing ‘clean’ versions if desired. Recruitment documents that will be outward-facing ‘as is’ (e.g., flyers for posting but not email text), require a ‘clean’ version for eResearch finalization process.
III. Standard Naming Convention for Study Documents
The standardized naming convention for uploads maintains consistency, provides historical documentation, and reduces errors. Tracked documents should be labeled with the ‘-tracked’ suffix.
Note: Exceptions to the standard naming convention can be made on a case-by-case basis at the discretion of IRBMED staff. Please reach out to IRBMED with any questions.
- Protocol-tracked
- Phone Script-tracked
- Email Template-tracked
- UM Flyer-tracked
- Flyer 18-40yo-tracked
- Flyer 41-80yo-tracked
- Social Media Kit-tracked
- Consent-tracked
Add a one- or two-word Concise Subtitle when there are multiple consents associated with the study.
For example:
- Consent – Control group-tracked
- Consent – Intervention group-tracked
For multi-site studies
- UM Consent-tracked
- Stanford Consent-tracked
For studies with minors
- Assent Script 10-13yo-tracked
- Assent Written 14-17yo-tracked
- Parental Permission-Tracked
For studies with multiple languages:
- English Consent-tracked
- English Assent script-tracked
- Spanish Consent-tracked
- Spanish Assent script-tracked
IV. Finalization & eResearch Watermark
Note: For Multi-Site Research/sIRB studies, IRBMED staff will not finalize/watermark the final documents since they are template documents to be used by all sites. Finalization/watermarks will be applied at the performance site level after review is complete.
IRBMED Staff label and clean all applicable Informed Consent Documents in question 10-1.1 and upload the final version for approval into their respective clean stack. The system finalizes documents into a .pdf with IRBMED approval watermark.
Study teams are responsible for providing ‘clean’ versions for finalization of Recruitment Documents in question 8-1.8.
Finalized documents may not be altered without review and approval via an amendment. For more information on Finalization and watermarking exceptions see Statement of Practice: IRBMED Finalization of Study Documents.
Questions?
Contact us at [email protected] or 734-763-4768 / (Fax 734-763-1234)
2800 Plymouth Road, Building 520, Room 3214, Ann Arbor, MI 48109-2800
A list of IRBMED staff is available in the Personnel Directory, or view the list of Regulatory Teams.
Edited By: [email protected]
Last Updated: October 15, 2021 8:30 AM

